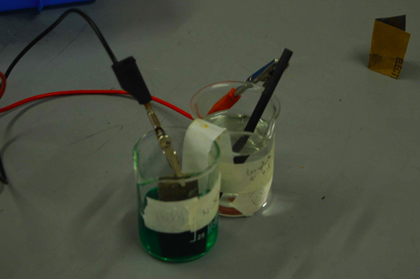
Example 1: Nickel - Hydrogen cell with a carbon electrode
C(s) | H+(aq), Cl-(aq) || Ni2+(aq), SO42-(aq) | Ni(s)
Cathode: 2H+ + 2e- --> H2 Ešr = + 0.00V
Anode: Ni --> Ni2+ + 2e- Ešr = + 0.26V
Balanced Net Equation: 2H+ + Ni --> H2 + Ni2+ Eš = +0.26V
The reaction is spontaneous; the hydrogen ions have a stronger pull on the electrons in nickel than nickel atoms themselves have, resulting in a reaction. The voltage detected by the voltmeter confirms there is a spontaneous reaction, as well as the presence of bubbles at the cathode.
Direction of electron flow: anode (-ve) --> cathode (+ve)
Descriptions of reactants and products:
HCl - liquid, colourless, clear, acidic odour
C - solid, black, dull, brittle
NiSO4 - liquid, green, clear, odourless
Ni - solid, bronze colour, lustrous, malleable
Product - Bubbles appear around the carbon electrode. A positive voltage was detected.
Identification of product: From the net equation, the products are H2 and Ni2+. Bubbles have been oberserved at the cathode, indicating the presence of H2(g). Ni2+ is present in the NiSO4 solution, indicating the presence of the ion.


Example 2: Copper(ii) - Aluminum cell
Cu(s) | Cu 2+(aq), SO4 2-(aq) || Al 3+(aq), Cl-(aq) | Al (s)
Cathode: 3[Cu2+ + 2e- -->Cu(s)] Eºr = + 0.34V
Anode: 2[Al(s) -->Al3+ + 3e-] Eºr = + 1.66V
Balanced Net Equation: 3Cu2+ + 2Al(s) --> Al3+ + Cu(s)
Eº = +2.00V
The reaction is spontaneous; the copper ions have a stronger pull on the electrons in aluminum than aluminum atoms themselves have, resulting in a reaction. The voltage detected by the voltmeter confirms there is a spontaneous reaction.
Direction of electron flow: anode (-ve) --> cathode (+ve)
Descriptions of reactants and products:
Cu(s) - solid, reddish-metalic in colour, malleable
CuSO4- blue translucent solution, odorless
Al(s)- solid, silvery-metalic solid, malleable
AlCl - clear translucent solution, odorless
Product - Copper sulphate solution pales slightly. A positive voltage was detected.
Identification of product: From the net equation, the products are solid copper and Al3+ ions . The paler colour of the copper sulphate solution suggests that the copper ions are being used up.


Applications Batteries
A battery is a portable, self-contained electrochemical power source that consists of one or more voltaic cells. For example, the common 1.5-V batteries used to power flashlights and many consumer electronic devices are single voltaic cells. Greater voltages can be achieved by using multiple voltaic cells in a single battery, as in the case in 12-V automotive batteries. Different applications require batteries with different properties. Some common batteries include:
- Lead-Acid Battery (12V): Consists of six voltaic cells in series, each producing 2V. The cathode of each cell consists of lead dioxide packed on a metal grid. The anode of each cell is composed of lead. Both electrodes are immersed in sulphuric acid. One advantage of a lead-acid battery is that it can be recharged. In an automobile the energy necessary for recharging the battery is provided by a generator driven by the engine.
- Alkaline Battery (1.55V): The most common primary battery. The anode of this battery consists of powdered zinc metal immobilized in a gel in contact with a concentrated solution of KOH. The cathode is a mixture of MnO2(s) and graphite, separated from the anode by a porous fabric. The battery is sealed in a steel can to reduce the risk of leakage of the concentrated KOH. The alkaline battery provides far superior performance over the older dry cells that were based on MnO2 and Zn as the electrochemically active substances.
- Nickel-Cadmium, Nickel-Metal Hydride, and Lithium-Ion Batteries The tremendous growth in high-power-demand portable electronic devices, such as cellular phones, notebook computers, and video recorders, has increased the demand for lightweight, readily recharged batteries. One of the most common rechargeable batteries is the Ni-Cad battery. There are drawbacks to Ni-Cad batteries. Cadmium is a toxic heavy metal. Its use increases the weight of batteries and provides an environmental hazard. Some of these problems have been alleviated by the development of NiMH batteries. The newest rechargeable battery to receive large use in consumer electronic devices is the Li-ion battery. It is because Li is a light element, Li-ion batteries can achieve a greater energy density than nickel-based batteries.